The Cooral® System has recently been approved though a De Novo clearance by the U.S. Food and Drug Administration (FDA).
The market clearance paves the way for prevention of one of the most severe side-effects of chemotherapy, Oral Mucositis (OM). OM is a severely debilitating condition characterized by erythema and ulcerations of the oral mucosa (National Cancer Institute) .
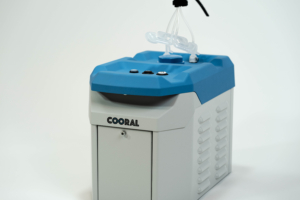
The Cooral® System offers a solution to improve the quality of life for patients undergoing chemotherapy by providing medical professionals a proven method to reduce the risk of oral mucositis (OM).
The System includes a device that circulates cold, sterile water through a mouthpiece fitted to the patient. Cryotherapy (cold therapy) is administered and reduces blood flow to a targeted area. Consequently, metabolism is slowed which reduces the risk of developing OM.

Improving quality of life
More information can for now be found on http://www.coolprevent.com a sister company of BrainCool that handles our pain management product portfolio.
